Del Mar Pharma is developing new drug candidates targeting orphan cancer indications representing market opportunities in the US$100s of millions in North America and potentially billions of dollars worldwide. We aim to develop products that will have a high impact in patient care and a high return for our investors.
In order to accelerate our development timelines and reduce technical risk, we leverage existing clinical and commercial data from a wide range of sources. This business model has been well validated and demonstrated to position for investor exit with significant upside, even prior to commercialization.
Key elements of our business strategy include:
- Leveraging existing human clinical data to support new regulatory filings with the U.S. FDA, Health Canada and the European Medicines Agency (EMEA).
- Advancing our products into orphan drug indications where patients are failing modern biologic or targeted therapy in order to obtain a guarantee of market exclusivity, attractive reimbursement, and high-likelihood of accelerated product approvals.
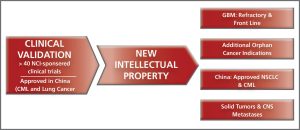
- Establishing new intellectual property around our product candidates, including use, manufacturing, chemical composition and mechanism patent claims.
- Obtaining commercial drug product from existing commercial manufacturers to accelerate entry into human clinical trials and reduce development costs.
- Investigating the molecular mechanism of our drug candidates in order to establish additional patent claims and also to identify patients most likely to benefit from therapy (personalized medicine approach).
- Commercializing our products through a combination of direct marketing and collaborative partnerships. For example, we target marketing our products to the small number of leading cancer centers in North America who specialize in the treatment of resistant orphan cancers and establishing sales and marketing partnerships for “large volume” tumor markets and in international markets including Europe & Asia.
- Generating near-term revenue by leveraging opportunities in markets where our drug candidates are already approved or can be readily registered.
- Operating on a “virtual” basis to minimize fixed-research costs while maintaining access to a highly-experienced and skilled workforce.